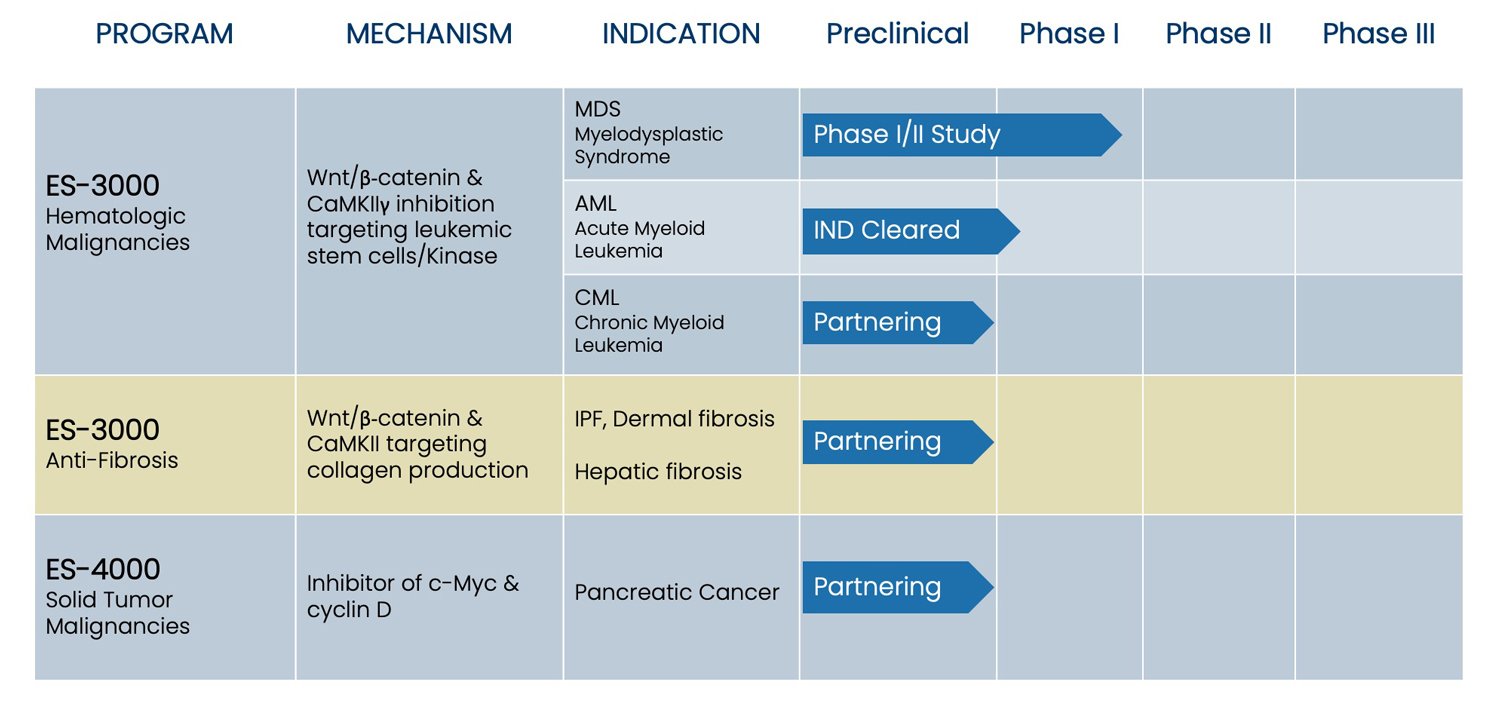
Pipeline
Development Pipeline
Our drug candidates target critical regulators of cancer, previously considered undruggable. These compounds not only kill proliferating cancer cells but also target cancer stem cells, which are a major cause of drug resistance and disease recurrence.
Clinical
Trials
Phase I/II Study for Myelodysplastic Syndrome (MDS)
ES-3000 is being investigated in a Phase I/II platform study for safety, pharmacokinetics and preliminary efficacy of experimental therapies versus standard of care in subjects with MDS.
Phase I/IIa Study for Acute Myeloid Leukemia (AML)
Escend is in preparation to initiate a Phase I/IIa study to investigate the, pharmacokinetics, safety and tolerability and preliminary efficacy of ES-3000 in subjects with relapsed or refractory AML. FDA has cleared our IND for this study.
ES-3000
Our lead drug candidate has demonstrated pre-clinical activity in acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS).
ES-3000 reduces leukemic stem cells through inhibition of Wnt/β-catenin cellular pathway and CaMKIIγ – a protein kinase. ES-3000 inhibits leukemic stem cells through inhibition of β-catenin and CaMKIIγ.
The US Food and Drug Administration (FDA) has cleared our Investigational New Drug (IND) application for a Phase I study in relapsed and refractory AML. Escend is in preparation to initiate the study.
The FDA Office of Orphan Drug Products (OODP) has granted orphan drug designation for ES-3000 for the treatment of acute myeloid leukemia (AML) and chronic myeloid leukemia (CML).
ES-3000 is being investigated in a Phase I/II, multi-arm platform study to compare the efficacy of experimental therapies versus standard of care in subjects with myelodysplasia.
ES-3000 for Acute Myeloid Leukemia (AML)
AML is the most common acute leukemia in adults and has the lowest survival rate. AML accounts for approximately 25% of all adult leukemias worldwide, with the highest incidence rates occurring in the United States, Europe and Australia. In the US, there are about 20,000 people each year that are diagnosed with AML.
AML is an aggressive cancer of the blood and bone marrow which prevents white blood cells from maturing, resulting in an accumulation of "blasts" which do not allow room for the normal blood cells. AML is a heterogeneous condition with a diverse set of genetic mutations. This heterogeneity extends to the leukemic stem cells (LSCs), which evolve to overcome selection pressures during disease progression. This results in multiple pools of LSCs within individual patients which may differ both phenotypically and molecularly. Leukemic stem cells are relatively resistant to current chemotherapeutic regimens and may be responsible for disease progression in AML.
Our objective is to develop ES-3000 for use with the current standard of care therapeutics in the treatment of AML to provide more durable remissions and a reduction in relapses.
ES-3000 for Myelodysplastic Syndrome (MDS)
MDS is a challenging pre-leukemic condition with limited treatment options and significant health, social and economic consequences for patients, their families and the health system. These patients are often highly dependent on blood transfusions and are commonly hospitalized with severe infections.
Unfortunately chemotherapy is rarely successful as a treatment option. High risk MDS invariably transforms to AML necessitating more intensive chemotherapy and/or allogenic bone marrow transplantation. Many patients are elderly and/or unfit. They often are unable to undergo such intensive interventions and they find that frequent hospital visits significantly disrupt their quality of life.
ES-3000 for Chronic Myeloid Leukemia (CML)
CML is caused by the Philadelphia chromosome (Ph) translocation t(9;22)(q34;q11) which generates a constitutively active tyrosine kinase, BCR-ABL1. Although CML is highly responsive to therapy with tyrosine kinase inhibitors (TKIs), such as Imatinib (Gleevec), which target BCR ABL1, about 33% of the treated patients do not achieve complete cytogenetic responses (CCyR) or are unable to tolerate drug-related toxicities.
In most CML patients for whom TKIs are largely effective, there is the pressing concern of low-level residual disease. As a result, only a small minority of patients can discontinue therapy, while the majority must continue therapy throughout their lives at considerable costs and sometimes enduring significant treatment associated side effects.
The potential for disease recurrence is due to the persistence of leukemia stem cells (LSCs) which are capable of surviving despite TKI inhibition of BCR ABL1.
Several survival pathways leading to TKI-resistance in CML have been identified, including the Wnt/β-catenin pathway which is the target of ES-3000. Although not required for the maintenance of normal human hematopoietic stem cells (HSC), the Wnt/β-catenin pathway is essential for the survival and proliferation of CML LSCs.
ES-4000
ES-4000 has demonstrated promise in the treatment of pancreatic cancer by targeting the over expression of c-Myc.
Recent studies have shown that increased c-Myc expression in pancreatic cancer correlates with poor prognosis and tumor progression. In cell culture and xenograft models, down regulation of c-Myc has been correlated to increased apoptosis and tumor regression.
ES-4000 strongly down regulates the expression of c-Myc. It is active at low nanomolar (nM) concentrations and more active than taxanes and camptothecins, both in vitro and in vivo. It has been demonstrated that c-Myc is over-expressed in hematologic and solid malignancies, particularly in pancreatic cancer.
ES-4000 was previously found active in Phase I clinical studies with heavily pretreated patients for metastatic breast cancer and melanoma. Inhibition of c-Myc overexpression by ES-4000 can be exploited as a novel therapy for pancreatic cancer.
About
Pancreatic
Cancer
Pancreatic Cancer is the fourth leading cause of cancer related deaths in the US. Approximately, 338,000 new cases of pancreatic cancer are estimated worldwide with more than 64,000 cases in the US in 2023, according to the American Cancer Society. Treatments include surgery, radiation and chemotherapy. Surgery is usually performed in about 20% of the newly diagnosed patients, mostly in conjunction with radiation and adjuvant chemotherapy.
Approved drugs include Tarceva, Abraxane and the most widely used chemotherapeutic drug, Gemcitabine, a nucleoside analog that affects DNA replication resulting in cell apoptosis.